|
|
|
|
|
|||||||||
|
|
|||||||||||||

UNIT 5 : EXPONENTIAL
& LOGARITHMIC FUNCTIONS
LESSON 2:
EXPONENTIAL EQUATIONS HOMEWORK QUESTIONS

1. Solve for x.
![]()
![]()
![]()
![]()
Solutions:
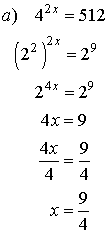
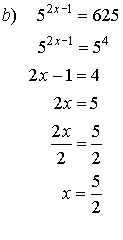
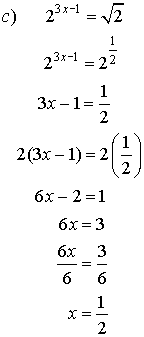
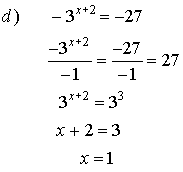
2. Solve for x.
![]()
![]()
![]()
![]()
Solutions:
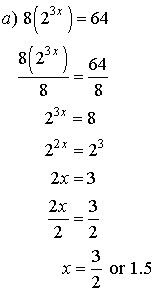
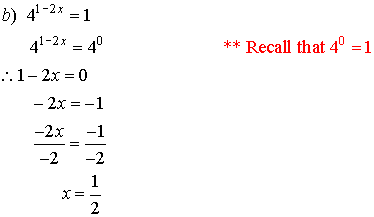
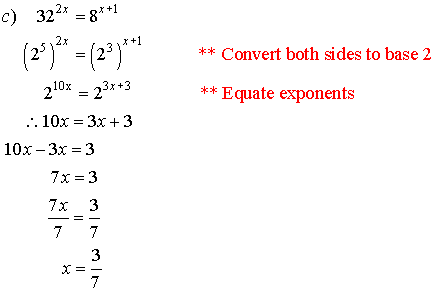
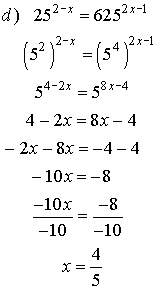
3. Solve for x.
![]()
![]()
![]()
![]()
Solutions:
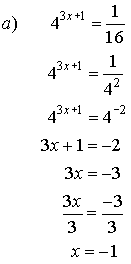
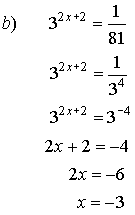
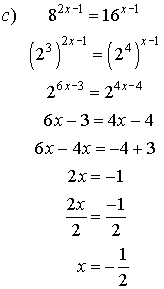
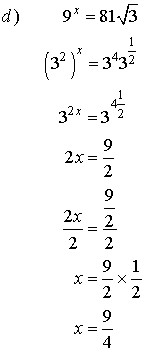
4. Solve for x.
![]()
![]()
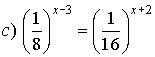
![]()
Solutions:
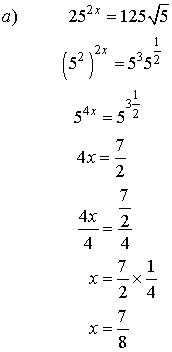
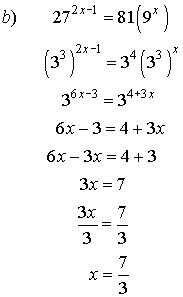
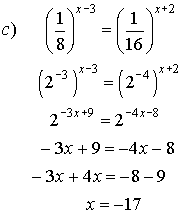
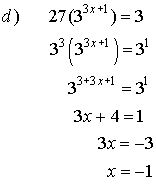
5. Solve for x.
![]()
![]()
![]()
![]()
Solutions:
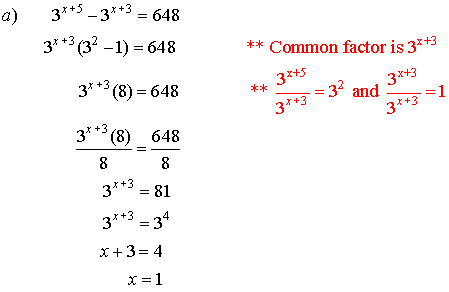
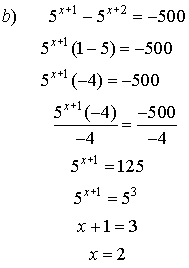


6. Solve for x.
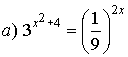
![]()
![]()
Solutions:
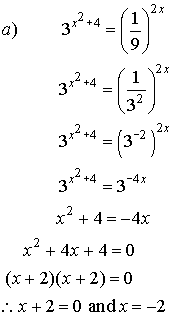
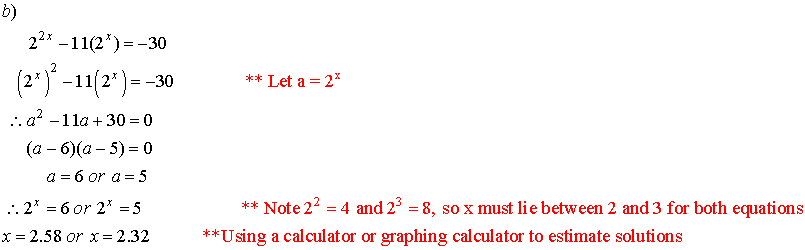
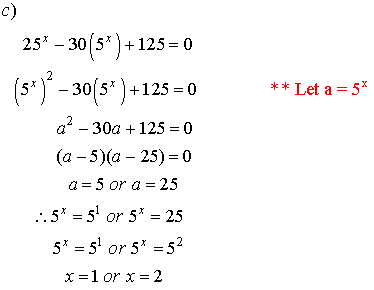
7. A
biologist grows a colony of bacteria in a Petri dish. Under ideal conditions, the doubling period is 3 h. How long will it take for the colony to grow
to 32 times its original size?
Recall:
A = ? A0 = ? t = ? d = 3 h
![]() , where
, where
·
A is
the number of bacteria after the given time frame
·
A0
is the starting number of bacteria
·
2 is
the growth factor
·
t is
the total time elapsed in the experiment
·
d is
the doubling period
Solution: In this problem, we are asked to find the time it takes to grow to 32
times its original size. We are not
told the exact starting and final numbers of bacteria. The key here is that whatever the starting
amount is, the final amount will be exactly 32 times larger. Therefore we let the starting amount be 1 g
. Hence the final amount will be 32
g.
We now
have the following information in the box
at right.
A = 32 g A0 = 1 g t = ? d = 3 h
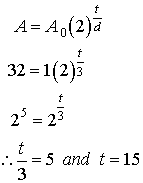
Therefore
it will take 15 h to grow to 32 times its original size.
8. The
half-life of radium is 1600 years. What
fraction of radium remains from a sample after 12 800 years?
Solution: Again let the starting mass be 1
g.
Recall:
A = ? A0 = 1 g t = 12 800 years h = 1 600 years
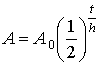 ,
where
,
where
·
A is
the mass remaining after the decay period
·
A0
is the original mass of radioactive material
·
½ is the decay factor
·
t is
the total time elapsed
·
h is
the half-life of the material
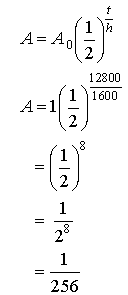
Therefore ![]() of the original
amount remains after 12 800 years.
of the original
amount remains after 12 800 years.
9. A bacteria culture doubles in size
every 20 min. How long will it take for
a sample of 10 bacteria to grow to 20 480?
Solution:
A = 20 480 A0 = 10 t = ? d = 20 min.
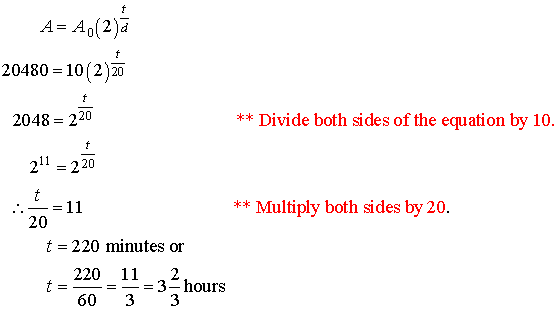
Therefore
it will take 3 2/3 hours.
10. A sample of uranium A decays to 3.125% of
its original mass after 2000 years.
Find its half-life.
Solution: Let the original mass be 100
g. Hence the remaining mass is 3.125 g,
leaving the information in the table below
A = 3.125 g A0 = 100g t = 2 000
years h = ?

Therefore
the half-life of uranium A is 400 years.